Chemical Reactions and Equations (Class 10 Science) Part 2

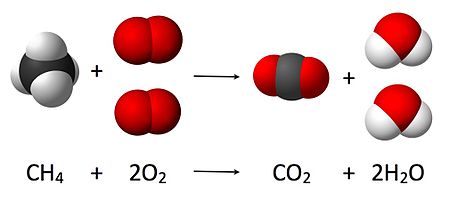
Balanced chemical equation A balanced chemical equation is that in which the total number of atoms of each element are equal on both sides of the equation. The balancing of a chemical equation is based on law of conservation of mass. According to this – “mass can neither be created nor be destroyed during a chemical reaction.” The method used for balancing chemical reaction is called hit and trial method as we make trials to balance the equation by using the smallest whole number coefficient. In this method the number of atoms of each element remains the same, before and after a chemical reaction. BALANCING A CHEMICAL EQUATION Several steps are involved in balancing a chemical equation. These steps are as follows :- Step (a) Writing unbalanced equation an...