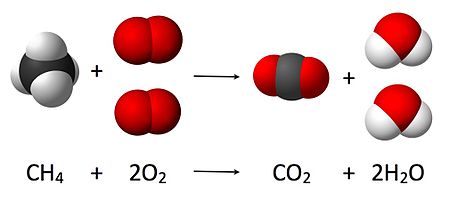
Corrosion // Rusting of iron

Corrosion It is the slow process of eating away metals by the reaction of atmospheric air and moisture, e.g. rusting of iron, formation of green coating over copper etc. PREVENTION OF CORROSION Rusting of iron is prevented by galvanising, by making alloys, painting, greasing or oiling and tin-plating and chrome plating . Explained below -- 1. Galvanisation - It is the process of coating iron and steel object with a thin layer of zinc. It is done by dipping the object in molten zinc. The galvanised article is protected against rusting even if the zinc coating is broken. 2. Alloying - It is the method of improving the properties of a metal by mixing the metal with another metal or non-metal. 3. Alloying of iron - Pure iron is very soft and stretches easilywhen hot. It is mixed with a small amount of carbon and it becomes hard and strong. Iron is mixed with many metals to form different types of alloys. For example, when iron is mix...