Types of chemical reaction
The
chemical reaction are classified into different classes depending upon the type
of chemocal changes taking place. These reactions are as follows :
1. Combination Reaction :-
A reaction in which two or more
reactants combine to form a single product, is called combination reaction,
e.g.
CaO(s)
+ H2O(l) → Ca(OH)2(aq) + Heat
C(s)
+ O2(g) → CO2(g)
2. Decomposition Reaction :-
A reaction in which a single reactants
breaks down to form two or more products, is known as decomposition reaction. This
reaction is opposite to combination reaction. e.g.
CaCO3(s) → CaO(s) + CO2(g)
2AgCl(s) → 2Ag(s)
+ Cl2(g)
2NaCl(l) → 2Na(s) + Cl2(g)
3. Exothermic and Endothermic reaction :-
Depending upon whether heat is evolved
or absorbed during a reaction, the reaction can be exothermic or endothermic.
(i)Exothermic reactions :- The reaction
which are accompanied by the evolution of heat, are called exothermic reactions
or the reaction in which heat is released along with the formation of products
are called exothermic reaction. e.g.
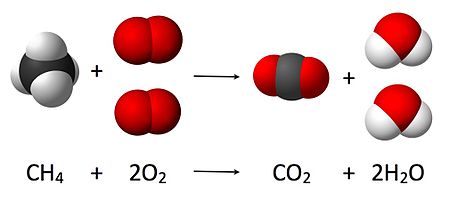
a)
Burning of
natural gas
CH4(g) + 2O2(g) → CO2(g)
b)
Burning of
Magnesium ribbon
2Mg(s) + O2(g) → 2MgO(s) + Heat
(ii) Endothermic reaction :- The reaction
which occur by the absorption of heat/energy are called endothermic reactions.
e.g.
6CO2(aq) + 12H2O(l) → C6H12O6
+ 6O2 + 6H2O
NH4Cl(s)
+ Heat → NH3(g) + HCl(g)
4. Displacement reaction :-
When a more reactive element displaces
less reactive elwmwnt from its compound, it is called displacement reaction.
this reaction is of two types :
(i) Single displacement reaction : It is a type
of chemical reaction where an element reacts with a compound and takes the
place of another element in that compound is called single displacement.
Zn(s) +
CuSO4(aq) → ZnSO4(aq) + Cu(s)
(ii) Double
displacement reaction : The reaction in which two different ions or
group of atoms in the reactant molecule are displaced by each other is called
double displacement reaction.
Na2SO4(aq)
+ BaCl2(aq) → BaSO4(s) +
2NaCl(aq)
5. Oxidation and Reduction reaction :-
OXIDATION :
It can be defined as
The
process in which oxygen is added to a substance.
Or
The
process in which hydrogen is removed from a substance.
Or
The
process in which a substance loss electrons.
e.g.
2H2S + O2 →
2S + 2H2O
2Cu + O2 → 2CuO
REDUCTION :
It can be defined as
The
process in which oxygen is removed from a substance.
Or
The
process in which hydrogen is added to a substance.
Or
The
process in which a substance gains electrons.
e.g.
2Na + H2 → 2NaH
Zn2+ + 2e− → Zn
Oxidising agents : The substance
which can bring about oxidation of other substances is called an oxidising
agent.
Reduction agent : The substance
which can bring about reduction of other substance is called a reducing agent.
Redox Reaction :-
Those reaction in which oxidation and
reduction take place simultaneously, are called redox reactions. e.g.
CuO + H2 →
Cu + H2O
In this reaction the copper oxide is losing
oxygen and is being reduced. Whereas oxygen is added to hydrogen and is being
oxidised.
Corrosion
The phenomenon due to which open surface of the
metals are slowly eaten away by the reaction of air, water, and chemicals
present in the atmosphere is called corrosion.
e.g.
Iron articles are shiny when new, but get coated with a reddish brown powder
when left for some time.
The
process of corrosion of iron is Calles rusting. The rusting of iron is a redox
reaction. the black coating on silver and the green coating on copper are other
examples of corrosion.
Painting,
Galvanising, electroplating are some of the methods to prevent corrosion.
Effects of Corrosion
(i)
Corrosion causes
damages to car bodies, bridges, iron railings, ships, and all objects made up
of metals, specially those which are made up of iron.
(ii)
Corrosion is a
wasteful process in most of cases. Every year tons of various metals especially
iron get wasted in the country.
Rancidity
It
is the process of slow oxidation of oil and fat present in the food material
resulting in the change of smell and taste in them. The method to prevent
rancidity are :
(i)
Keeping food
material in air tight containers.
(ii)
Refrigeration of
cooked food at low temperature.
(iii)
Packing of food
items like potato wafers etc in packets containing nitrogen gas instead of air.
(iv)
Avoid keeping
cooked food and food materials in direct
sunlight.
Part 1 Part 2